



Prevention of Contaminations with Clostridia in Silages Using Inoculants
Clostridia are endospore-forming anaerobic Gram-positive bacteria which ferment carbohydrates, lactic acid (LA), as well as amino acids and proteins, thus causing problems such as reduction in feeding value and the production of biogenic amines (McDonald, 1981;Oude Elferink et al., 1999).The main sources of Clostridia are soil, crops and silages, which can contaminate the milk and cheeses. However, McDonald (1981) stated that the presence of Clostridia in silage is mainly related to soil contamination since the Clostridia population on the plants is very low. The silage smell of silage contaminated with Clostridia is a vomit-like caused by higher butyric acid (BA) content. Therefore, besides of the pungent odor, a major problem on the farm is refusing silage intake by animals. Clostridia can be divided in three main physiological groups (adapted from McDonald, 1981) as listed below (examples on the right side):
a) saccharolytic e.g. Cl. butyricum
b) proteolytic, and e.g. Cl. sporogenes
c) saccharo-proteolytic e.g. Cl. perfringens
This classification says a lot about their detrimental effect on silages: they metabolize sugars (also LA) and protein (and amino acids).
At a pH value of 4.5 the growth of the undesirable bacteria is relatively slow (Wilkinson, 2005). A pH value below 4.5 is considered generally as an insurance to keep Clostridia under control. Silages like corn silage, for example, are less affected by Clostridial fermentation because of higher cut height and the concomitant lower contamination with soil, as well as the commonly very fast acidification to silage pH values below 4.0. Modern methods of ensiling as bailing have the disadvantage that the pH drop is not so deep, however normally the crop will be harvested at higher DM content, diminishing the risk for Clostridial fermentation but not that for Listeria (1, 2) and mold and mycotoxin contamination (Acosta Aragón and Rodrigues, 2009) due to the difficult compacting of a drier material.
If the pH value drop is not deep enough, the pH value can subsequently increase again due to the fermentation of LA to weaker acids like acetic and butyric acid, with a concomitant degradation of proteins and amino acids (Wilkinson, 2005). The risk of secondary (for example, butyric) fermentation is increased in crops (Wilkinson, 2005; Spiekers, 2006):
- wet (DM<20%),
- with relatively low sugar content (< 30g of water soluble carbohydrates/ kg of fresh weight),
- with relatively high buffering capacity (> 400 mEq/ kg DM), and/ or
- with low concentration of nitrates (< 10g NO3/ kg total N)
- with high contamination with soil, caused mainly by low height cut and reflected in increased ash content in the laboratory analysis, should be added to above cited risks.
A maximal value for ash content would be of 4.5 and 10.0% in DM for corn and grass silages respectively. Nußbaum (2011) considers that the increase in 1% of sand decreases the energy density in 0.1 MJ NEL/ kg DM.
Clostridial silages are characterized by a high BA content of more than 3 g/kg DM (Spiekers, 2011), a high pH (over pH 5 in low DM silages), and a high ammonia and amine content (Flythe and Russel (2004). Ensiling methods that cause a rapid and sufficient drop in silage pH will help to prevent the development of such Clostridial silage, because, as for Enterobacteria, Clostridia are inhibited at low pH. Clostridia are more susceptible to a low availability of water (low aw-value) than LA bacteria (Huchet et al., 1995). This means, decreasing the moisture/ aw-value of a crop, such as by wilting to a higher DM content, can be a way of selectively inhibiting Clostridia (Wieringa, 1958).
Clostridia will also be inhibited by nitrite or compounds that are degraded in silage to nitrite (Spoelstra, 1985). Kaiser and Weiß (2007) consider an optimal value for NO3 of 4.4–13.0g/ kg DM, and less than 4.4 a disadvantage for the ensiling process and an increased risk for Clostridia contamination.
Clostridial spores present in silage are transferred to milk via feces and fecal contamination of the udder. The acid-tolerant Clostridium tyrobutyricum is the most relevant species for the dairy industry. This BA fermentation not only counteracts lactic acid fermentation in silage and cheese, but it is also responsible for significant gas production, causing a cheese defect called “late blowing” in hard and semi-hard cheeses (Klijn et al., 1995, Wilkinson, 2005).
Some Clostridia can cause serious health problems, for example, Clostridium botulinum and botulism, which can be deadly for cattle. However, C. botulinum cannot survive at low pH values which are common in well-fermented silage (Oude Elferink, 1999). Incidences of animal botulism caused by silage contaminated with C. botulinum could nearly always be attributed to the presence of a cadaver in the silage (Kehler and Scholz, 1996).
Material and methods
A pool of the results from 54 field trials with grass silage was used. According to their relatively low dry matter content 16 trials, 9 not treated silages and 7 treated with the commercial product Biomin® BioStabil Plus (BSP, blend of homo- and heterofermentative lactic acid bacteria), were selected to evaluate the activity of Clostridia mainly through the silage butyric acid content.
Samples were collected after 3 (for pH value determined by a portable pH meter) and 45 (for full silage quality analysis in local certified laboratories) days after ensiling using a core sampler. The dry matter content of the selected silages varied from 19.6 to 26.0 %. Parameters like pH value (3 and 45 days of ensiling; 5.99 and 5.09 respectively), as well as LA and BA (15.17 and 15.27 g/ kg DM respectively) and ammonia N from total N (10.65 %) were collected. Statistical parameters like average, standard deviations, and minimal and maximal values as well as Pearson correlations (2-tailed) and media comparison (T-Student) were calculated using the statistical package SPSS 18.0.
Results
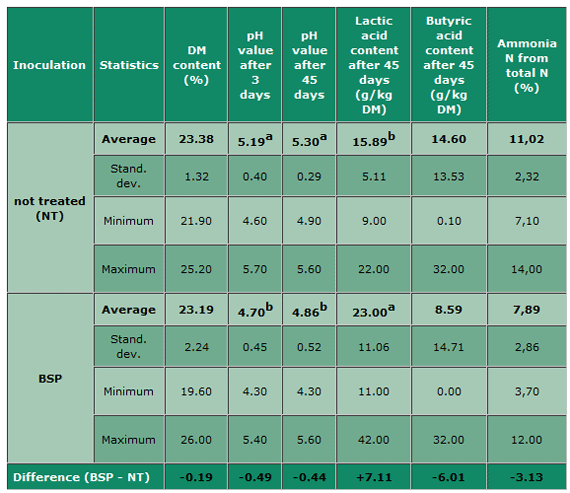
In Table 1 a comparison between the treated and not treated silages is shown. Statistical significances were found only in the pH value after 3 and 45 days, and the lactic acid content. However, there are numerical differences also in BA and ANtN, being higher in the not treated silages (+6.01g/ kg DM and +3.13 %) than in the silages treated with BSP. It corresponds very well with the higher LA content and the concomitant lower pH values after 3 and 45 days, meaning that the Clostridial activity was decreased due to the higher acidity, producing less BA and ANtN.
Table 1: Comparison between treated with Biomin® BioStabil Plus and not treated silage
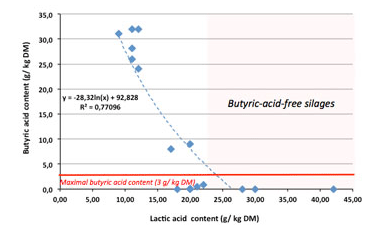
According to the graphically presented results in Figure 1, there is a negative correlation between the LA and the BA. The logarithmic equation which explains such a correlation shows a negative B value and a high r2 (0.771). A practical interpretation of the results is that silages with more than 16-17g of LA/ kg DM are below the critical value for BA given by the DLG for good silages (less than 3g/ kg DM). If the production of LA goes over 22-23g, then silages would be BA-free. Of course, those are orientation values and cannot be generalized.
The actions to decrease the Clostridia contaminations in silages head mainly the decrease of the ash content (contamination with soil) in the harvested material and the inhibition of the Clostridia growth. All those actions have to begin on the field, go through the harvest and finish with an appropriate storage.
Conclusions
- Clostridia can affect the silage quality by diminishing its palatability, as well as energy and protein content
- The most effective control for Clostridia is a quick and deep decrease of pH (below 4.5)
- Wet or dirty silage, low nitrite content and/ or relatively sugar contents can lead to a Clostridial fermentation
- The control for Clostridia must be a package beginning on the field, going through a clean harvest and ending with the use of adequate silage additives
- The butyric acid is the main product in a Clostridial fermentation and is highly negatively correlated with the pH value and lactic acid content
June 2012



